Arsip
Altering poultry sex ratios
Chickens, like most animals, typically produce equal numbers of males and females. But this natural sex ratio doesn’t always work in the poultry industry’s economic favor. A University of Georgia researcher is working on ways to skew the chicken’s sex ratio to help the industry streamline production and make more money.
Chickens are big business in Georgia, worth $4.9 bln in 2008, or 41% of the state’s total agricultural value. For the broiler sector of the poultry industry, the females are less profitable. On average, male broilers weigh half a pound more than females at market age, and they eat 5% less feed. However, in the egg-laying sector, the females are prized over males, obviously, because males can’t grow up to produce eggs.
Kristen Navara, a poultry scientist with the UGA College of Agricultural and Environmental Sciences, is trying to determine how to control avian sex ratios.
“In nature, it is a necessary strategy to adjust offspring sex in relation to the environment,” she said. “Humans, rodents, birds all skew sex ratios. It is clear females need the ability to adjust offspring for the environment where they will be born or hatched into.”
Navara has recently studied skewed sex ratios in hamsters and humans in relation to day length. She is now looking for the mechanism that can control the ratios in poultry and finches. She’s using hormones, particularly corticosterone, to find that mechanism.
Injecting female birds with a burst of corticosterone just before ovulation produced a sex ratio skewed toward males, or 81%. She believes she can flip the ratio to favour males or females using hormones or aggravates, which stop the secretion of corticosterone.
Sara Beth Pinson, a graduate student in Navara’s lab, is coordinating studies to determine the optimal dose of corticosterone to produce the desired result. They are also testing different durations of the hormone treatment to determine how long-term treatments affect offspring sex. Research results could be available in 6 months.
This research “is something the industry has been looking for for years,” said Mike Lacy, head of the CAES poultry science department. “The US poultry and egg industry funded Dr. Navara to do this research because it is something the industry is very interested in.”
It is important to note that no chickens used for food are given hormones. Navara’s research is only using hormones to discover the mechanism. “Broilers are not treated with hormones. “So far, the hormone injections seem to work, but what we want to do is find the mechanism the hormone is working through and then produce a non-hormonal treatment for the birds. That is the optimal way to go,” she said.
SOURCE: WorldPoutry.net
Brooding and rearing baby chicks
Baby chicks are really quite easy to raise. With a few pieces of equipment and a small place to put them, success in brooding and rearing is virtually assured. During this period of the bird’s life, the most important needs are for warmth, protection, feed, and water. When growing chicks of any species-chickens, turkeys, pheasants, or almost any other production bird-each of these aspects must be considered.
Natural vs. artificial brooding
In nature, chicks hatch after 2 to 4 weeks of incubation by the parents, most often the hen. The hatched chicks provide the stimulus to the hen to change her work from incubating eggs to brooding young. This form of brooding chicks is the easiest if only a few chicks are raised because the mother hen does all the work.
Hens that are “good mothers” include Rhode Island Red, New Hampshire, Plymouth Rock, Cochins, and Silkies. Under natural brooding, chicks can easily be fostered under a broody hen at night, and she will raise them as her own even if they are pheasants, turkeys, quail, or waterfowl.
When broody hens are not available, or large numbers of chicks are to be raised, artificial brooding is necessary. Chicks will perform equally well under artificial or natural brooding, providing they are precocial; that is, able to walk and feed themselves within hours of hatching, as baby chickens are.
Novice growers are not advised to try artificial brooding for altricial chicks; that is, chicks such as pigeons, doves, finches, and parrots that remain in the nest to be cared for and fed by the parents. Many of these chicks are naked, blind, and unable to walk for several weeks after hatching and require around-the-clock care and feeding.
Pemilihan Pengawet Produk Olahan Daging
Oleh Edi Suryanto
 Untuk menghindari kerusakan, maka daging perlu diawetkan. Pengawetan daging dapat dilakukan dengan penambahan bahan pengawet yang termasuk dalam Bahan Tambahan Pangan (BTP). Namun masyarakat sekarang merasa ketakutan apabila mendengar istilah bahan pengawet atau bahan kimia yang dapat menimbulkan efek negatif bagi tubuh. Padahal, ketakutan ini tidak perlu terjadi. BTP sebenarnya adalah bahan aditif yang mengandung senyawa-senyawa kimia, misalnya natrium klorida, senyawa nitrit/nitrat, senyawa phosphate, dan lainnya yang telah diijinkan penggunaannya. Namun yang menjadi pertanyaan apa jenis pengawet yang cocok untuk produk olahan daging, bagaimana dengan keamanan dan ambang batas penggunaan, dan amankah bahan pengawet tersebut bagi kesehatan konsumen?
Untuk menghindari kerusakan, maka daging perlu diawetkan. Pengawetan daging dapat dilakukan dengan penambahan bahan pengawet yang termasuk dalam Bahan Tambahan Pangan (BTP). Namun masyarakat sekarang merasa ketakutan apabila mendengar istilah bahan pengawet atau bahan kimia yang dapat menimbulkan efek negatif bagi tubuh. Padahal, ketakutan ini tidak perlu terjadi. BTP sebenarnya adalah bahan aditif yang mengandung senyawa-senyawa kimia, misalnya natrium klorida, senyawa nitrit/nitrat, senyawa phosphate, dan lainnya yang telah diijinkan penggunaannya. Namun yang menjadi pertanyaan apa jenis pengawet yang cocok untuk produk olahan daging, bagaimana dengan keamanan dan ambang batas penggunaan, dan amankah bahan pengawet tersebut bagi kesehatan konsumen?
Bahan-bahan yang umum digunakan untuk pengawetan produk olahan daging antara lain adalah 1) garam (sodium chloride), 2) alkaline phosphates (sodium tripolyphosphate), 3) sweetener seperti dextrose, sukrosa dan sorbitol, 4) sodium atau potassium nitrite digabungkan dengan sodium atau potassium erythorbate atau ascorbate, 5) sodium laktat atau potassium lactate, 6) sodium acetate dan diacetate, 7) liquid smoke, 8) antioxidan seperti butylated hydroxy anisole (BHA), butylated hydroxy toluene (BHT) propyl gallate (PG), alpha tocopherols. Terdapat pula beberapa asam yang digunakan untuk menghambat pertumbuhan mikroorganisme pada karkas unggas. Karkas ayam yang dicelupkan dalam larutan asam laktat atau asam sitrat mempunyai masa simpan yang lebih lama.
Competitive Exclusion and Vaccination for Reduction of Salmonella Enteritidis Contamination of Eggs
The Salmonella enteritidis (SE) pilot project (Schlosser et al. 1995) showed that the risk of eggs being contaminated with SE could be reduced by proper house cleanout and disinfection, rodent control, biosecurity, and by housing SE-free chicks and pullets. As a result, the focus for SE risk reduction in the commercial egg industry has been on adoption of voluntary egg quality assurance (QA) programs (e.g. the Pennsylvania and California Egg Quality Assurance Programs, and others modeled after them) which stipulate specific standards for management practices. QA programs appear to have been successful for reducing the incidence of SE in eggs in regions of the United States where SE has been a problem.
New methods for controlling SE in poultry have been developed, such as competitive exclusion of SE in the intestinal tract and SE vaccination. Presently, the number of commercial products available for either of these options is limited, but commercial offerings no doubt will increase if these approaches to SE control prove effective in the field.
Competitive exclusion (CE) cultures contain microflora that can live in the digestive tract of a chicken. In fact, these cultures are derived from intestinal samples taken from mature chickens. When established in a bird’s intestinal tract, the CE culture hinders colonization by salmonellae bacteria. One advantage of CE cultures is that they exclude many species of Salmonella, not just Salmonella enteritidis. The protection they offer is immediate. To be effective, a CE culture must be administered before a bird is exposed to salmonellae because the culture will not reliably eliminate salmonellae which already reside in the bird’s intestinal tract. Field trials have demonstrated that salmonellae contamination of processed broiler carcasses was reduced when chicks from hatcheries having low environmental levels of salmonellae were treated with a CE culture (Blankenship et al. 1993). By preventing early colonization of chicks by salmonellae, the CE culture appears to forestall buildup of the bacteria in the growing environment, leading to lower levels of contamination when flocks reach market weight. Currently, there is little published scientific data for commercial layers regarding the efficacy of CE cultures but it stands to reason that similar control of salmonellae, including SE, could be achieved during pullet growout and at other specific times in the life of a hen.
A chicken is especially vulnerable to salmonellae colonization when its digestive tract does not have an established population of normal microflora, as occurs at hatch, after administration of antibiotics, and apparently also during feed deprivation. A hen is very susceptible to SE infection during the feed withdrawal phase of an induced molt.
In environments where SE is present, competitive exclusion cultures would have their greatest potential to control SE infection of commercial layers if applied specifically at times when the flock is unusually susceptible to SE colonization. These times would be at hatch and housing, after antibiotic treatment, and perhaps during induced molt feed withdrawal and any other occasion which might cause the flock not to feed. Once mature microbial populations have been established in the gut of a chicken, CE cultures offer little additional protection against salmonellae. This may explain why in the example mentioned above, salmonellae contamination of processed broiler carcasses was not entirely eliminated in flocks treated with a CE culture. One cannot expect that CE products would give commercial layers total protection from SE.
SE vaccines operate differently than competitive exclusion products in that they cause a chicken to develop antibodies which protect it specifically against SE, as opposed to other Salmonella species. Immunization makes a bird more resistant to SE than it would otherwise be in normal circumstances, and the protection lasts for months. Protection obtained from SE vaccination develops gradually as the chicken’s immune system forms antibodies. To develop immune protection by the start of egg laying, SE vaccine must be administered to pullets during the growing period. A booster vaccination during an induced molt may also be advisable.
SE vaccination cannot guarantee total protection against SE because any immune system can be overwhelmed by high doses of an infectious agent, but field trials suggest promising reductions of SE occurrence in vaccinated flocks (Schlosser et al. 1995). SE vaccination would be particularly useful in situations where an SE population might persist after cleanout of an SE-positive house, or when an SE challenge is likely, as in an SE-positive complex where rodents can travel between houses.
Neither competitive exclusion nor SE vaccination will eliminate the need for a comprehensive egg quality assurance program because the best way to ensure that eggs do not become SE contaminated is to make sure
hens are never exposed to SE. In environments where SE might be present, both competitive exclusion and SE vaccination, properly applied, would be useful components of a QA program to minimize risk of SE contamination of eggs.
References:
Blankenship, L.C., J. S. Bailey, N.A. Cox, N.J. Stern, R. Brewer, and O. Williams, 1993. Two-step mucosal competitive exclusion flora treatment to diminish Salmonellae in commercial broiler chickens. Poultry Science 72:1667-1672.
Schlosser, W., D. Henzler, J. Mason, S. Hurd, S. Trock, W. Sischo, D. Kradel, and A. Hogue, 1995. Salmonella enteritidis Pilot Project Progress Report. U.S. Government Printing, Washington, DC.
By A. Bruce Webster, Extension Poultry Scientist
Poultry Tips newsletter – College of Agricultural and Environmental Sciences
The University of Georgia Cooperative Extension Service
Transporting Broiler Chickens Could Spread Antibiotic-Resistant Organisms
Researchers at the Johns Hopkins Bloomberg School of Public Health have found evidence of a novel pathway for potential human exposure to antibiotic-resistant bacteria from intensively raised poultry—driving behind the trucks transporting broiler chickens from farm to slaughterhouse. A study by the Hopkins researchers found increased levels of pathogenic bacteria, both susceptible and drug-resistant, on surfaces and in the air inside cars traveling behind trucks that carry broiler chickens. The study is the first to look at exposure to antibiotic-resistant bacteria from the transportation of poultry. The findings are published in the first issue of the Journal of Infection and Public Health.
Typically, broiler chickens are transported in open crates on the back of flatbed trucks with no effective barrier to prevent release of pathogens into the environment. Previous studies have reported that these crates become contaminated with feces and bacteria.
The new study was conducted on the Delmarva Peninsula—a coastal region shared by Maryland, Delaware and Virginia, which has one of the highest densities of broiler chickens per acre in the United States. Ana M. Rule, PhD, a research associate in the Bloomberg School’s Department of Environmental Health Sciences, along with professor Ellen K. Silbergeld, PhD, and Sean L. Evans collected air and surface samples from cars driving two to three car lengths behind the poultry trucks for a distance of 17 miles. The cars were driven with both air conditioners and fans turned off and with the windows fully opened. Air samples collected inside the cars, showed increased concentrations of bacteria (including antibiotic-resistant strains) that could be inhaled. The same bacteria were also found deposited on a soda can inside the car and on the outside door handle, where they could potentially be touched.
“We were expecting to find some antibiotic-resistant organisms since it’s pretty clear that the transportation conditions for these chickens are not closed or contained,” Rule said. “Our study shows that there is a real exposure potential, especially during the summer months, when people are driving with the windows down; the summer is also a time of very heavy traffic in Delmarva by vacationers driving to the shore resorts.”
The strains of bacteria collected were found to be resistant to three antimicrobial drugs widely used to treat bacterial infections in people. These drugs are approved by the U.S. Food and Drug Administration for use as feed additives for broiler poultry. The study findings were also consistent with other studies on antibiotic resistance in poultry flocks and poultry products.
According to the authors, the findings support the need for further exposure characterization, and attention to improving methods of biosecurity in poultry production, especially for regions of high density farming such as the Delmarva Peninsula.
Support for the study came via the Johns Hopkins Center for a Livable Future’s Innovation Grant Program.
Factors Affecting Egg Quality
Many factors affect egg quality. Sometimes the cause is not a single factor but a combination of factors. A few of these problems can be prevented or reduced by good hen management. Producers should remember that genetics, feed quality and environment play a role in egg quality. The most reliable factor is age. A young pullet produces smaller eggs with strong egg shells and albumen that stands high. As the hen ages, the shells thin, and the albumen begins to weaken and run. Hens can be molted to induce another egg cycle, which will improve egg quality, or they will need to be replaced with young pullets.
The following charts summarize factors that may affect egg quality and suggest corrective measures. As more emphasis is placed on egg quality, it is important that all possible defects be eliminated. When defects are found, consult the chart for possible causes and solutions.
Baca selengkapnya…
Effect of Summer Heat Stress on Poultry Breeding Stock
As the hot summer months approach producers´ attention is turned to management methods designed to maintain productivity during elevated ambient temperatures. For broiler and turkey meat producers, getting the birds to continue eating and efficiently converting their feed source to weight gain is the overall objective. The effects of heat stress have been well documented in relation to feed consumption, weight gain and house efficiency in broilers. In extreme heat situations, keeping birds alive becomes the most critical element, especially in older meat-type birds.
For producers of broiler breeders, the volume of feed the birds consume is restricted, so even during elevated temperatures the birds will often still consume the feed provided to them. This is especially true for broiler breeder males that will generally eat all the feed provided them in less than an hour during both summer and winter months. During this time of the year, however, the birds´ energy needs are reduced, and therefore, they do not require as much feed for maintenance as they do during the winter months. The problem with breeders is maintaining egg production, fertility, hatchability and ultimately the number of quality chicks produced. We, as an industry, have come a long way in the utilization of quality equipment in the breeder houses and therefore in reducing in house temperature spikes. Twenty years ago it was estimated that there was an average 15% drop in fertility in broiler breeders during the summer months. Due to improvements in housing, the reductions in fertility due to heat stress may not be so dramatic today. Nevertheless, the industry generally sees the lowest fertility and hatchability during the hot summer months. Baca selengkapnya…
Early Feed Intake and Bird Performance
mportance of Early Feed Intake
Feed intake is the single most important factor in determining growth rate of commercial broilers and turkeys. The data in Table 1 were obtained from industry sources and show that birds consuming feed the fastest weighed the most at processing and converted feed best. These data show what experienced growers have known for some time — flocks that do the best tend to be active and consume starter feeds quickly.
It is also important to realize that feed intake is most important in the youngest birds. Figure 1 illustrates this fact. Most of the energy and nutrients consumed by birds younger than four weeks goes toward growth. After four weeks the majority of energy and nutrients goes toward maintaining the bird’s body. This means that if energy and nutrients are restricted early in the bird’s life, it will likely never catch up to birds that were provided a good start.
In addition, flocks in which the majority of birds start well tend to be relatively uniform in size, making management and optimum results easier. Flocks with the highest feed intake will almost always have the highest average daily gain and weigh most at processing.
Reducing Energy Costs in Poultry Houses
Reducing Energy Costs in Poultry Houses
Calcium in Laying Rations: How to Sample Feed and Interpret Analysis
The purpose of this fact sheet is to demonstrate how to sample laying hen rations and interpret the calcium levels found in the feed. The results of a study of feed from 24 Saskatchewan leghorn flocks are used to help illustrate the reliability of calcium testing.
How to Sample Feed
Because the amount of calcium can vary from one sample of feed to the next, it is important to take a good feed sample:
1. Take a small sample of feed, approximately 0.20 Kg or 0.5 lbs., each day for four days. The four
samples should be taken from the feed hopper, not the troughs or pans that the birds are eating
from.
2. Mix the four samples together to give one large sample. By mixing together feed from four days, you
are more certain that the feed did not come from just one corner of the feed bin.
3. Send the sample to an accredited lab and tell the lab that the sample is a layer ration.
4. If the calcium level reported by the lab appears to be abnormally low or high, ask the lab to double
check the results. Most labs keep samples for several weeks after analyzing them and should be able
to re-analyze them if necessary.
Also check the level of ash reported for the feed. Because ash represents the total level of minerals in the feed, it should be high when the calcium is high and low when the calcium is low. If the calcium reported for the sample is abnormal but the ash appears to be normal, you may want to question the accuracy of the test. In a feed sample with a normal level of calcium, there will be 11% to 13% ash.
Can You Trust Calcium Analysis ?
Analysis for calcium has a reputation for producing variable results. When the calcium tested in a feed is too high or low, it is often said, "Oh, that sample must have gotten an extra piece of limestone" or "…that sample must be missing a piece of oyster shell". If the feed is properly sampled according to the method described above, these concerns can be greatly reduced.
To determine how variable calcium testing can be, two different batches of feed were tested repeatedly for calcium. From each batch, 32 small feed samples were taken and eight large samples were then formed by mixing the small ones together in groups of four. One of the feeds was formulated to contain 3.85% calcium and all of the calcium came from ground limestone. The other feed was formulated to contain 4.0% calcium, with 75% of the calcium coming from oyster shell. One sample of each feed was submitted to the feed lab each month for eight months and the calcium levels tested.
Overall, the calcium analysis was accurate. Seven of the eight limestone feed samples was within +/- 0.35% of the formulated value while six of the eight oyster shell feed samples were within +/-0.3%. Only one sample of each type of feed tested had a calcium level low enough to be of concern. The one abnormal sample of the limestone feed initially tested at 2.95% calcium but tested at 3.85% when re-analyzed. The sample of unusual oyster shell feed tested at 2.94% calcium when first analyzed and was still 3.28% calcium when double checked. This particular sample actually was "missing" a piece of oyster shell and was lower in calcium than the other samples. While this one sample was out, it was possible to get a reasonable estimate of calcium in 15 of the 16 samples tested between the two feeds.
Producers wanting to be absolutely certain of the calcium level in the feed should test a sample taken on the farm and ask the feed company to analyze its sample of the same load of feed. By analyzing two different samples taken at different places, it is possible to rule out any errors in feed sampling or testing.
The table below will assist in interpreting your test results.
|
Calcium Level |
Symptoms or Concerns |
| < 3.0% | Poor shell thickness; cage layer fatigue; increased feed intake and possibly hemorrhagic fatty liver syndrome |
| 3.0 to 3.5% | Thinner shells and weaker bones if fed for a prolonged period or feed intake is depressed |
| 3.5 to 4.5% | Good shell quality and bone strength |
| 4.5 to 5.0% | No improvement in shell quality or bone strength; higher feed cost |
| > 5.0% | May dilute feed if such high levels are unplanned; possible sign of poor feed mill calibration or quality control |
Adapted from Saskatchewan Poultry Pointers
By Carlyle Bennett, Business Development Specialist
Manitoba Agriculture, Food & Rural Initiatives – Livestock Knowledge Centre
Published 08/20/2008
Source: Manitoba Agriculture, Food & Rural Initiatives Technorati Tags: Perkuliahan
Ascites in poultry
The ascites syndrome in broiler flocks has been increasing at an alarming rate, and this condition has become one of the leading causes of mortality and whole carcass condemnations throughout the world. Ascites represents a spectrum of physiological and metabolic changes leading to the excess accumulation of fluid in abdominal cavity. These changes occur in response to a number of dietary, environmental and genetic factors. Improvements in growth performance and decreases in mortality rates, as well as benefits in alleviating ascites have been observed in recent trials with BIOMIN’s acidifier product Biotronic® SE.
Definition:
The term "ascites" actually refers to the fluid accumulation in abdominal cavity (or so called "waterbelly"). The disease is more scientifically known as pulmonary hypertension syndrome. Ascites is most commonly diagnosed at 4 – 5 weeks of age. Total mortality due to ascites is higher in the male parent lines, which have the capability of faster growth and higher muscle deposition compared to the female lines (Dewil et al., 1996).
Avian Influenza Factsheet: 12 informative tips
1) First and foremost, the H5N1 virus causing problems in Asia, Europe, and Africa has not been detected anywhere in North, Central or South America at this time.
2) Properly cooked poultry meat is safe to eat in any case as cooking destroys the virus. It is recommended that poultry meat should be cooked to an internal temperature of at least 180 degrees Fahrenheit throughout each piece.
3) A National Surveillance Program is being done in the USA. All poultry flocks are tested for Influenza before processing. Again, H5N1 has never been detected in US poultry.
4) Biosecurity measures in commercial poultry operations throughout the US are at maximum levels.
5) Biosecurity measures, surveillance efforts, and veterinary practices in place in the commercial poultry industry;
a) greatly reduce the possibility that disease will enter into commercial poultry flocks
b) would quickly detect presence of this disease
c) would result in immediate quarantine and depopulation of a poultry farm where H5N1 was detected
and stringent containment, cleaning and disinfection measures would occur.
6) It is possible that migrating birds may spread the virus out from Asia this summer or fall. Surveillance testing is continuing and H5N1 was not detected in thousands of samples collected in Africa this past winter. Surveillance efforts of migratory birds in the USA to date have also not detected this virus.
7) All deaths from H5N1 virus in foreign countries to date have been attributed to prolonged or direct contact with infected poultry or due to backyard or market processing practices that are not used in the commercial poultry industry in the U.S. Only one case of “possible” human to human spread is known, but there has been no sustained human to human transmission.
8) The primary concern about the H5N1 virus that has been reported in the news is the possibility of mutation that would enable readily and sustainable transmission from person to person. This mutation has not been detected in the virus to date. Furthermore, even if such a mutation were to occur, it does not mean that a pandemic is imminent.
9) Since the first recorded death from H5N1 avian influenza virus strain in humans in 1997, there have been about 205 confirmed known human infections with 115 confirmed deaths throughout the world.
10) While these deaths are tragic, it should be recognized that there are 32,000-35,000 deaths each year in the U.S. alone from human influenza virus.
11) Basic hygiene is recommended by health experts to prevent contracting influenza (practices such as covering your mouth when sneezing or coughing and then washing your hands afterwards).
12) Considerable research is being conducted throughout the world on new vaccine development and other technologies that will help in preventing and/or slowing the spread of H5N1. It is believed these new vaccines and technologies will lessen the possibility of an H5N1 pandemic.
By Dr. Walter Bottje, Director Center of Excellence for Poultry Science, University of Arkansas
Published 07/03/2008
Source: Univ.of Arkansas Division of Agriculture factsheet Technorati Tags: Perkuliahan
The Only Good Broiler Breeder Egg is a Fertilized Egg
The main goal of broiler breeder management is producing eggs. However, the only good broiler breeder egg is a fertilized egg. Fertility, the percentage of eggs laid that are fertilized, is very important in poultry production. If an egg is not fertilized, then, of course, it will not contain an embryo and will not hatch. Simply put, "Hatchability can never be better than fertility."
Hatchability is around eight percentage points lower than fertility because many chick embryos are usually lost during incubation. For example, even if 93 percent of the eggs laid are fertilized, then under normal incubation conditions only 85 percent of the eggs will hatch. This example illustrates how fertility must be very good to get above average hatchability and hatch bonus pay.
Breeders need to be kept under ideal conditions for maximum life of flock fertility. The chicken’s reproductive system is very sensitive to the bird’s environment, and under poor conditions the reproductive system will dwindle. For example, the environment can cause a rooster’s testes to increase or decrease in size by several hundred fold. But, before we can understand which management factors influence fertility, we must first examine the fascinating process of fertilization in poultry.
Fertilization in any animal depends on production of eggs from the female and sperm from the male. A problem with either sperm or egg production can decrease fertility. The rooster’s reproductive system is simple when compared to humans or other mammals. The rooster does not have a prostate gland or any of the accessory reproductive glands. Like all other animals, chicken sperm carry the genetic material from the rooster and are produced within the testes. The rooster has two very large testicles within the abdominal cavity on each side of the backbone. After sperm leave the testes, they enter the epididymis, where they gain the ability to swim. Next, the sperm enter the vas deferens, where they are stored until the rooster mates with a hen.
Sperm formation takes about 15 days. The rooster’s semen contains around 5 billion sperm per cc, about 40 times as much as that of a human. Once a rooster is mature and if he is maintained properly, he will manufacture about 35,000 sperm every second of his life. However, just like the males of many animal species, the fertilizing potential of roosters varies, even within a flock. For example, some roosters are extremely fertile and create a maximum number of quality sperm; other roosters are subfertile and do not make enough good sperm. This variation in rooster quality is caused by management, environment, nutrition, and genetics.
The hen does not produce nearly as many eggs as the rooster produces sperm, but during her 40 weeks of production, the broiler breeder hen lays about 180 eggs. Egg formation requires about 25 hours. Since egg formation requires more than 24 hours, even the best hens cannot lay an egg every day in succession throughout their productive life. As is the case with roosters, some hens are more productive than others, and management has a major impact on variability among hens.
The hen’s reproductive system can be divided into two major components: the ovary and the oviduct. The ovary produces the egg yolk. The oviduct adds the white, shell membranes, and shell to the yolk to complete egg formation.
The hen has only one ovary, which is on the left side of her abdomen. The ovary has several thousand ova (egg yolks) in different stages of development and looks like a bunch of grapes. Very immature yolks contain only genetic material from the hen, and as the yolks grow to around 1 mm in diameter, they become white. If the hen is managed properly, many of these developing egg yolks will mature in about 19 days into large, 35 mm, yellow yolks. As the egg yolk develops it will get water, sugars, fats, proteins, vitamins, and minerals from the hen’s blood. These are all necessary for the embryo to develop. The egg yolk is surrounded by the perivitelline membrane. This keeps all of these nutrients in a ball-shaped package. One particularly visible region of the perivitelline membrane is the germinal disc. The germinal disc is a small white dot about half the size of a pencil eraser on the surface of the yellow egg yolk. Fertilization takes place here, and embryonic development begins.
When the egg yolk is mature, it leaves the ovary, and within 20 minutes it is captured by the infundibulum, the first part of the oviduct. Here fertilization takes place. Following mating, sperm enter the hen’s oviduct and are stored within sperm storage glands. Only sperm that can swim will enter these sperm storage sites. These glands can store more than half a million sperm. Sperm can remain alive in these glands and fertilize eggs for up to 3 weeks.
A hen will have maximum fertility for only about 3 to 4 days after one mating. For this reason, the male-to-female ratio in a flock must be enough to ensure mating of every hen every 3 days or so. Sperm do not break through the eggshell. Instead they travel up the oviduct to the infundibulum to join with the egg yolk.
The sperm bind to the perivitelline membrane and make a hole as they enter the egg. Hundreds of sperm may enter the yolk. As a matter of fact, the more sperm that enter the yolk, the more likely the egg will be fertilized. Around 30 sperm must enter the egg near the germinal disc to insure a 95 percent chance of fertilization. While it is true that only one sperm is necessary to fertilize an egg, the probability of an egg’s being fertilized by only one sperm’s reaching and penetrating it is very low.
After about 15 minutes, the yolk leaves the infundibulum (fertilized or not) and receives the egg white, shell membranes, and shell over the next several hours from the magnum, isthmus, and uterus sections of the oviduct. When the hen lays a fertilized egg, the chick embryo has already developed for about 25 hours into approximately 20,000 embryonic cells and is a live, breathing organism. If this fertilized egg is handled properly before and during incubation, a healthy baby chick is the result.
By Dr. Chris McDaniel, Associate Professor, Poultry Science Department, Mississippi State University – MSU Cares Information Sheet – Mississippi State University Extension Service
Published 06/26/2008
Source: Mississippi State University Extension Service Technorati Tags: Perkuliahan
Managing Today’s Broiler Breeder Female
Managing the modern broiler breeder female so that she will produce a large number of high quality hatching eggs is a delicate combination of both art and science. Over the past few decades, broiler breeders have undergone intensive selection for faster growth rate, increased yield and improved feed conversion. Although these traits are measured at the broiler level, they impact the breeder hen in ways we often do not consider. The objective with broiler breeders is to have them consume an “ideal” amount of nutrients within a given time period to produce a bird whose weight, body condition and frame allow the reproductive organs to mature and function at their best. How do we combine art and science to manage the sexual maturation of today’s broiler breeder female?
Can Eggshell Quality be Determined by Shell Color?
Problem
* Is there a relationship between eggshell color and eggshell quality?
* Is computer-based image analysis technology a reliable indicator of shell color?
Introduction
Throughout the world, preference for a shell color in table eggs has differed and is based mainly on the visual appeal of the egg. For example, in North America the preference is for white-shelled eggs while in Asia the preference is for brown-shelled eggs. Shell color has very little to do with the nutritional value of a table egg. But is there a possible relationship between eggshell quality and shell color?
Previous studies have shown that there is a correlation between shell color and shell quality in eggs of a single strain. The overall finding was that darker brown eggs had a higher shell quality than lighter brown eggs. Shell quality was determined by specific gravity, egg weight loss (due to storage) and shell strength. While an association was found, it was too small to conclude that shell color was a more reliable measure of shell strength than specific gravity. Also, the measurement of shell color used in these past studies was subjective and may have been biased.
Approach
To determine if a relationship exists between eggshell color and eggshell quality, four different strains of broiler breeders were used. Each strain was subjected to three different feeding programs. The four strains of broiler breeders used in this study were the Cobb 500, Shaver Starbro, Hubbard Hi-Y and Avian 24K. To maintain anonymity, these strains were randomly and irrespectively assigned a code of either W, X, Y, or Z. These birds were part of a larger study (by Dr. Frank Robinson) testing the effects of different feed treatments on four strains of broiler breeders. The three feeding programs were ad libitum (AL), fast feed (FF) and slow feed (SF).
At 50 weeks of age, eggs were collected and weighed over a one-week period. The eggs were weighed and their specific gravity was determined. Shell color was determined using image analysis software, Northern Exposure (Empix Imaging Inc.). An egg was placed in a small (2.5 cm) aluminum cylinder with its large end up. A video camera placed directly above the egg records a picture of the egg and projects this image onto a computer monitor. The image was saved in black and white and stored for future measurements. The computer recorded a total of 818 images. Shell color was assessed as an average gray value on a scale of 0 to 255; with 0 being black and 255 being white. Average gray value was referred to as shell color units (scu) for the purposes of this study (Figure 1). After the images were taken, each egg was opened, the yolk and albumen were emptied and the shell was rinsed under warm water and placed in egg flats to dry so that they can be weighed.
Specific gravity was not influenced by feed treatment. This was unexpected, as the AL fed birds laid the largest eggs of the three groups (Figure 2), we assumed that they would have a lower specific gravity. The AL fed birds also produced the lightest eggshell color than either the FF or SF groups (Figure 3). There were strain differences in shell color with strain W producing the darkest colored eggshells and strain X producing the lightest. However, differences in shell color across strains or feeding programs could not be consistently correlated to any specific shell quality parameter (i.e. specific gravity, shell weight or egg weight loss).
Image analysis was an effective tool for measuring shell color in broiler breeder eggs. Strain appeared to have a great influence on shell color but it was not the only factor involved. There are several experiments that are being proposed to further study the effects of shell color on shell quality.

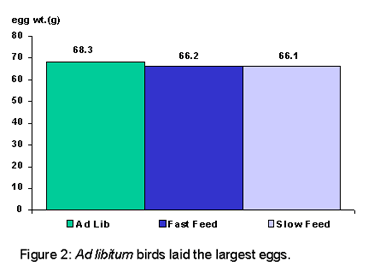
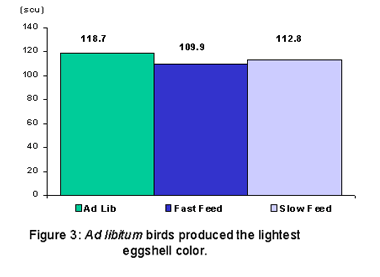
By Nancy Joseph (Source: Poultry Research Centre News – Vol. 7 No. 2)
Published on the Government of Alberta Agriculture and Rural Development website
Published 08/12/2008
Source: Govt. of Alberta Agriculture and Rural Development Technorati Tags: Perkuliahan


























Komentar Anda